
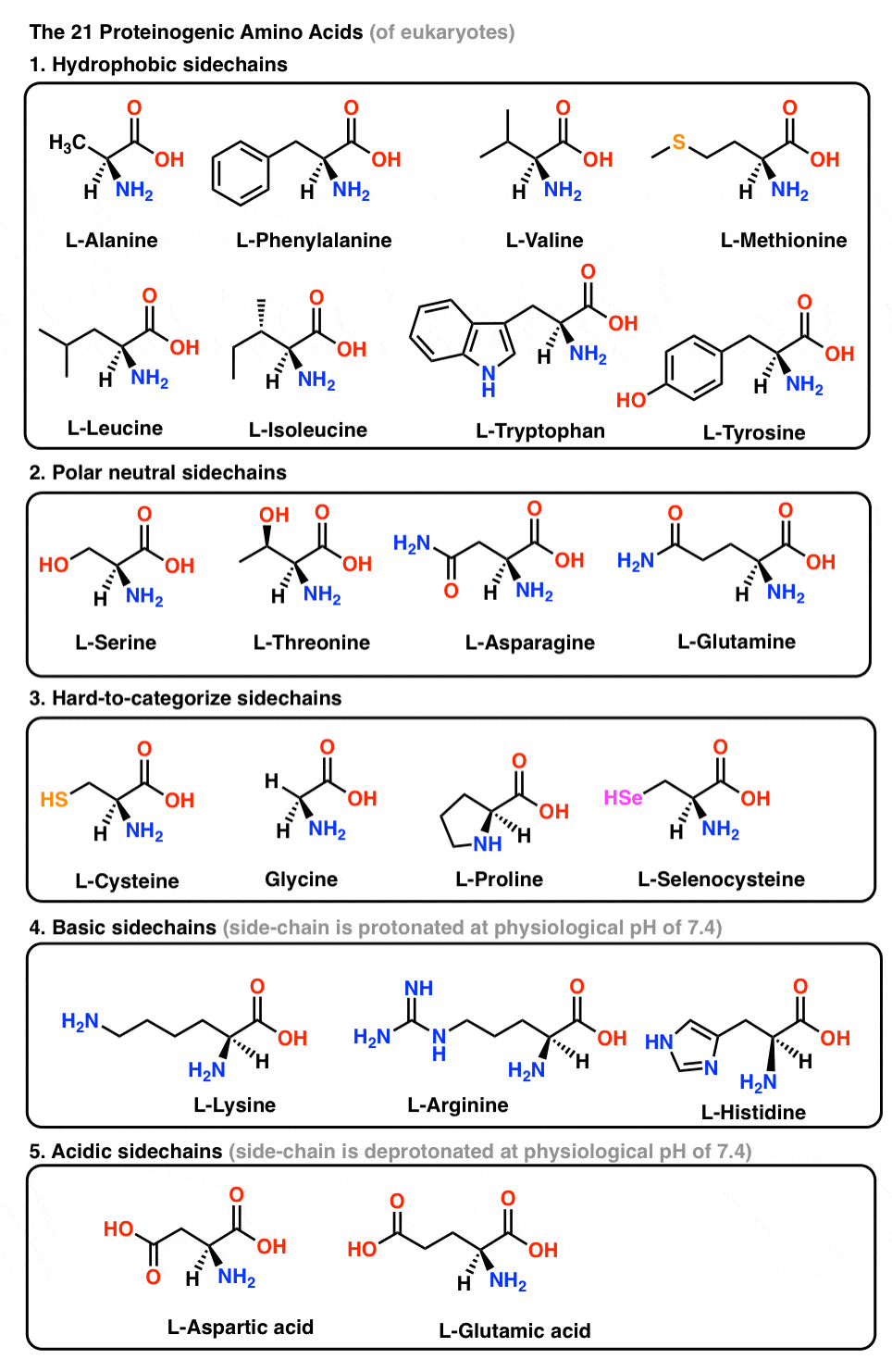
This conformational restriction brought by CH/π interaction produced an inhibitory structure, in which the C-terminal amide- benzyl group fits the chymotrypsin S 1 site and the hydrophobic core binds to the S 2 site. Their spatial proximity was evidenced by 400 MHz 1 H-nmr measurements, observing large upfield shifts of proton signals of D-Xaa- alkyl and nuclear Overhauser effect (NOE) enhancements between the D-Xaa- alkyl and Phe-phenyl groups.
The D-Xaa-alkyl and Phe-phenyl groups resulted in a formation of the hydrophobic core due to the side-chain-side-chain CH/π interaction. A novel type of conformationally restricted peptides with the structure of H-D-Xaa-Phe-NH-CH 2-C 6H 5 has been developed as inhibitors of serine proteinase chymotrypsin.


 0 kommentar(er)
0 kommentar(er)
